Guidance: Applying for permission to import medicinal cannabis and cannabis-related substances
Guidance for completing an application for permission to import medicinal cannabis and cannabis-related substances.
Introduction
The importation of medicinal cannabis substances subject to regulation 5 of the Customs (Prohibited Imports) Regulations 1956 is prohibited unless the importer holds a licence and permit issued by the Narcotics Control Section (NCS). A permit is required for each consignment that is imported whereas licenses are issued annually. A licence to import must be obtained before a permit can be granted. Information on obtaining a licence is available separately from the Office of Drug Control (ODC) website.
Permits are not granted to individuals for the purpose of obtaining medications for personal use. If you are an individual wanting to access medications that are prohibited imports you should consult your doctor and refer to the Special Access Scheme on the TGA website.
Permitted purposes for importing medicinal cannabis
An application may be made to import medicinal cannabis products for the following purposes:
- Authorised Prescriber (AP) Scheme
- Special Access Scheme (SAS)
- Clinical Trials
- Animal Studies
- Laboratory or analytical testing
- Cultivation under an ODC Medicinal Cannabis Permit.
Application requirements
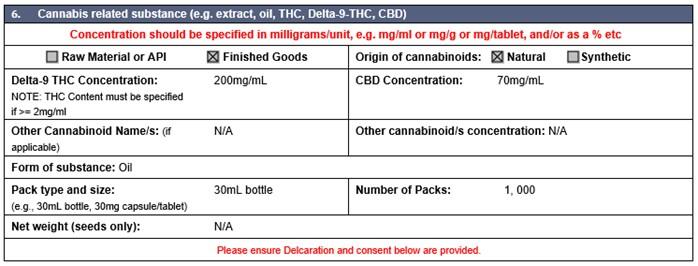
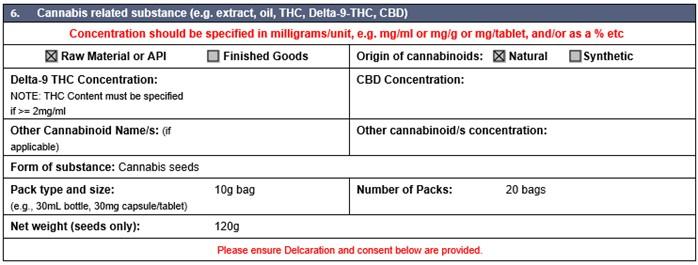
Medicinal cannabis and its products (such as cannabis oil, extracts and tinctures) are regulated as medicines in Australia and are prescription only substances. Only delta-9-tetrahydrocannabinol is permitted for importation into Australia. All other associated isomers of tetrahydrocannabinol (THC) are not permitted for import for use in Australia.
Importers must specify the form of THC being imported when completing import permits and are now required to specify whether the cannabinoids are of natural or synthetic origin.
To apply for a permit to import controlled substances the application form titled ‘Application for permission to import cannabis and cannabis-related substances’ must be submitted to NCS. The guidance provided here will assist you in completing and submitting the application form.
Completing the application form
The table below identifies the required information for completing the permit application form:
Submitting your application
You can submit your application in the following ways:
| Narcotics Control Section Office of Drug Control GPO Box 9848 Canberra ACT 2601 | |
| NCS@health.gov.au |
The NCS endeavours to process applications for permits within 20 business days from the date of receipt of a viable application form and the required supporting documentation.
While a very high proportion of applications are processed within 10 days, there will be times where high demand for permits may result in slightly longer processing times. Application forms that contain incomplete or incorrect information will be returned to you for amendment, resulting in delays in processing.
It is the responsibility of the importer to ensure that the triplicate copy of the permit is completed at the time of importation and the hardcopy returned to NCS.
It is responsibility of the importer to return the endorsed triplicate copy to NCS no later than 14 working days after the importation has occurred. Failure to comply with this condition may result in cancellation of import licenses.
Unused or expired permits must be returned within 14 days.